Research: Structural Colors in Plants
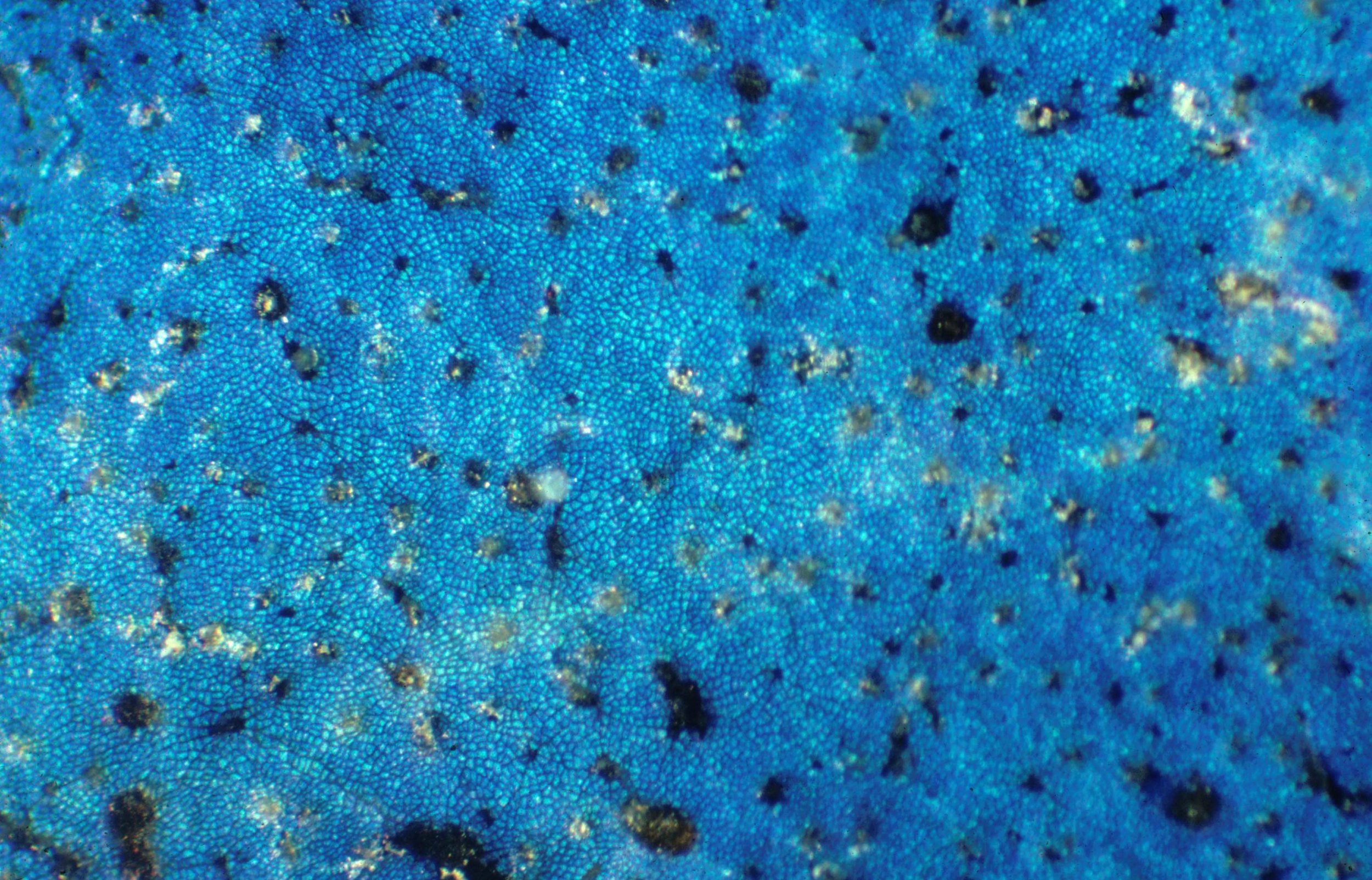
I discovered several species of understory plants in Malaysia with metallic blue colors, produced by physical structures and not pigments. I studied this phenomenon with collaborators over the next 40 years, in Malaysia but also in Latin America (particularly Costa Rica) and in all tropical forests I visited. This led to some collaboration with physical scientists working in the area of nano-photonics and spurred the interests of present scientists working actively in this field today:
Heather Whitney, University of Bristol
Silvia Vignolini, University of Cambridge
Barbara Glover, Plant Science University of Cambridge
Important Articles
-
Nanostructures for Coloration (Organisms other than Animals)
by Ille C. Gebeshuber and David W. Lee
in Encyclopedia of Nanotechnology, 2014
Structural colors refer to colors generated by minuscule structures, with the characteristic dimension of the structures on the order of the wavelength of the visible light (i.e., some tens up to hundreds of nanometers). Examples for structural colors are the colors of CDs and DVDs, the colors of soap bubbles, or oil films on water (thin films), or the colors of certain butterfly wings (e.g., photonic crystals), and even plants. Tiny wax crystals in the blue spruce scatter the light (Tyndall scattering), resulting in the blue hue. Thin films in tropical understory plants and diffraction gratings in hibiscus and tulip flowers are just some more examples of the amazing variety of natural nanostructures that are the basis for coloration in some plants. This entry reviews the physics behind structural colors, lists plants, microorganisms, and virus species with nanostructures responsible for their coloration, and also touches upon the multifunctionality of materials in organisms, nanobioconvergence as an emergent science, and biomimetics as a promising field for knowledge transfer from biology to engineering and the arts.
-
Silica nanoparticles aid in structural leaf coloration in the Malaysian tropical rainforest understorey herb Mapania caudata
by Greg Strout, Scott D. Russell, Drew P. Pulsifer, Sema Erten, Akhlesh Lakhtakia and David W. Lee
in Annals of Botany 112, 2013
Background and Aims
Blue-green iridescence in the tropical rainforest understorey sedge Mapania caudata creates structural coloration in its leaves through a novel photonic mechanism. Known structures in plants producing iridescent blues consist of altered cellulose layering within cell walls and in special bodies, and thylakoid membranes in specialized plastids. This study was undertaken in order to determine the origin of leaf iridescence in this plant with particular attention to nano-scale components contributing to this coloration.
-
Plant Tissue Optics: Micro- and Nanostructures
by David W. Lee
in Biomimetics and Bioinspiration, Proc of SPIE Vol. 7401, 2009
ABSTRACT
Plants have evolved unusual tissue optical properties, not surprising as creatures of light. These are astonishingly sophisticated, involving both micro- and nanostructures. Microstructures refract, scatter, and channel light in plant tissues, to produce concentrations and gradients of light within, and to remove undesired portions of the electromagnetic spectrum. Nanostructures use the different refractive indices of both cellulosic walls and bi-lipid membranes to interfere with light, multiple layers producing intense constructive coloration and reduced fluxes within tissues. In a tropical sedge now under analysis, structures may include silica. Recently discovered surface diffraction gratings produce strong directionally sensitive coloration that assist in pollinator visitation. Although some of these properties have obvious applications, most await appreciation by creative scientists to produce new useful devices.
-
Structural Fruit Coloration in Delarbrea Michieana (Araliaceae)
by David W. Lee, George T. Taylor, and Anthony K. Irvine
in International Journal of Plant Sciences 161.2, 2000
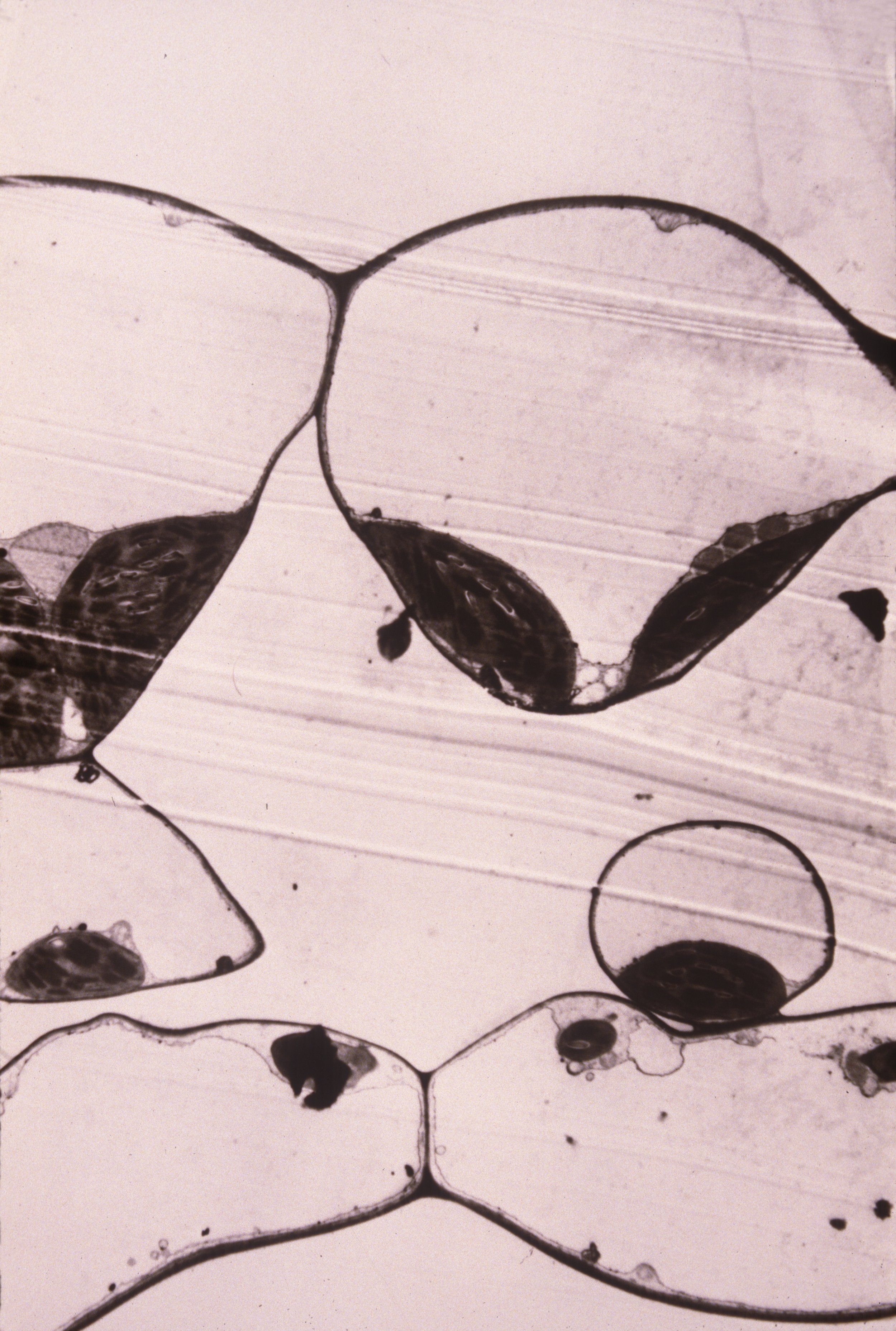
The fruits of Delarbrea michieana (F. Muell.) F. Muell. (Araliaceae), a small understory tree of north Queensland rain forests, are a brilliant iridescent blue. The color of nearly all fleshy fruits is the result of pigmentation. The selective absorption of light scattered through the epidermis and cortex produces the yellows, reds, blues, and violet-blacks of fruits; these colors are presumably signals to aid in dispersal of these fruits. Blue color, which is present in ca. 5%–7% of fleshy fruits (Wheelright and Janson 1985), is caused by anthocyanins, which are modified by metallic cations or by copigmentation. When crushed, such fruits yield a juice that is colored pink-red by these pigments. However, the fruits of numerous taxa within Elaeocarpus (Elaeocarpaceae), such as Elaeocarpus angustifolius Blume, produce brilliant blue coloration from constructive interference (Corner 1988), which is caused by structures (iridisomes) within the epidermis (Lee 1991). Although such a basis for fruit coloration may be exceedingly rare, in this paper we describe the structural basis for iridescent blue fruit color in D. michieana and speculate on its phylogenetic and ecological significance.
-
Effects of Irradiance and Spectral Quality on Leaf Structure and Function in Seedlings of Two Southeast Asian Hopea (Dipterocarpaceae) species
by David W. Lee, Steven F. Oberbauer, Paulette Johnson, Baskaran Krishnapilay, Marzalina Mansor, Haris Mohamad, and Son Kheong Yap
in American Journal of Botany 87.4, 2000
ABSTRACT
We studied the development of leaf characters in two Southeast Asian dipterocarp forest trees under different photosynthetic photon flux densities (PFD) and spectral qualities (red to far-red, R:FR). The two species, Hopea helferi and H. odorata, are taxonomically closely related but differ in their ecological requirements; H. helferi is more drought tolerant and H. odorata more shade tolerant. Seedlings were grown in replicated shadehouse treatments of differing PFD and R:FR. We measured or calculated (1) leaf and tissue thicknesses; (2) mesophyll parenchyma, air space, and lignified tissue volumes; (3) mesophyll air volumes (Vmes/Asurf) and surfaces (Ames/Asurf); (4) palisade cell length and width; (5) chlorophyll/cm2 and a/ b; (6) leaf absorption; and (7) attenuance/absorbance at 652 and 550 nm. These characters varied in response to light conditions in both taxa. Characters were predominantly affected by PFD, and R:FR slightly influenced many characters. Leaf characters of H. odorata were more plastic in response to treatment conditions. Characters were correlated with each other in a complex fashion. Variation in leaf anatomy is most likely a consequence of increasing leaf thickness in both taxa, which may increase mechanical strength and defense against herbivory in more exposed environments. Variation in leaf optical properties was most likely affected by pigment photo-bleaching in treatments of more intense PFD and was not correlated with Amax. The greater plasticity of leaf responses in H. odorata helps explain the acclimation over the range of light conditions encountered by this shade-tolerant taxon. The dense layer of scales on the leaf undersurface and other anatomical characters in H. helferi reduced gas exchange and growth in this drought-tolerant tree.
-
The biology of rudraksha
by David W. Lee
in Current Science 75.1, 1998
Rudraska, used throughout India and Southeast Asia in religious jewellery, is the stony endocarp of a tree distributed from northern Australia to southern Nepal. This article summarizes its biology, particularly recent research on the remarkable fruit color. The iridescent blue colour is caused by a remarkable structure, an ‘iridosome’. It is secreted by the epidermal cell, and is located above the plasmalemma and beneath the adaxial wall. Cellulosic layers within the iridosome constructively interfere with blue wavelengths, causing an intense colour production at 439 nm. This colour persists in seescing fruits and may enhance their dispersal. The transparency of the cuticle at longer wavelengths allows photosynthesis to occur in the fleshy green exocarp tissuue, enhancing the carbon balance of the tree. More research will certainly reveal the evolution of this remarkable phenomenon, as well as the origins of the rudraksha’s cultural use.
-
Physical and Ultrastructural Basis of Blue Leaf Iridescence in Two Neotropical Ferns
by Rita M. Graham, David W. Lee, Knut Norstog
in American Journal of Botany, 80.2, 1993
ABSTRACT
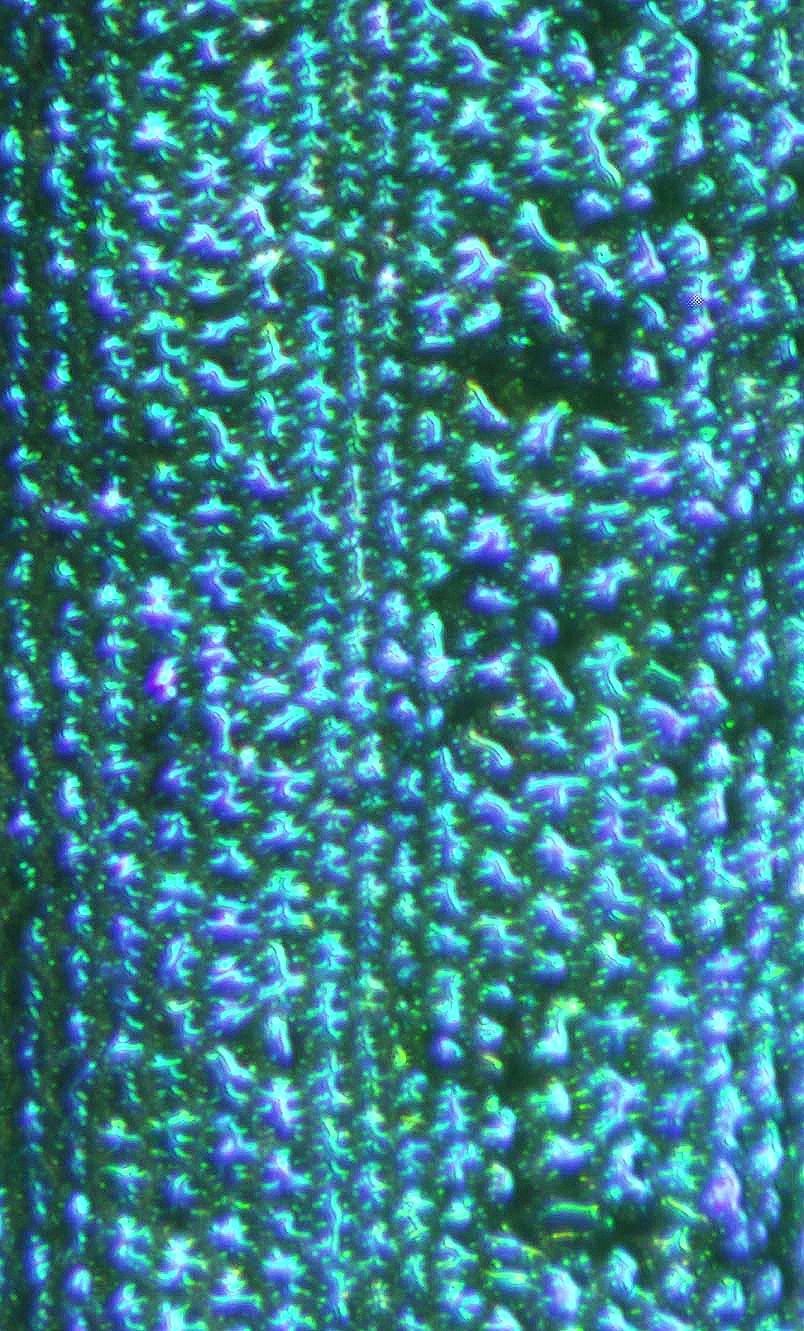
Iridescent blue leaf coloration in two neotropical ferns, Danaea nodosa (L.) Sm. (Marattiaceae) and Trichomanes elegans L. C. Rich. (Hymenophyllaceae), is caused by thin film constructive interference. The ultrastructural basis for the film in D. nodosa is multiple layers of cellulose microfibrils in the adaxial cell walls of the adaxial epidermis. The apparent helicoidal arrangement of the fibrils is analogous to similar color production in arthropods. In T. elegans the blue-green coloration is caused by the remarkably uniform thickness and arrangement of grana in specialized chloroplasts adjacent to the adaxial wall of the adaxial epidermis. The selective advantage of this color production, if any, is unknown but apparently different from that previously studied in Selaginella.
-
Ultrastructural basis and function of iridescent blue colour of fruits in Elaeocarpus
by David W. Lee
in Nature 349, 1991
Iridescent colour, caused by physical effects (thin-film interference, diffraction and Tyndall scattering), is relatively common in animals but exceedingly rare among plants. Some benthic marine algae produce blue to violet iridescence, and the upper leaf surfaces of a few vascular plants from the shady environments of humid tropical forests are iridescent blue. Blue fruit colour has been assumed to be caused by anthocyanins. A survey of such fruits (26 species in 18 genera) in Costa Rica, India, Florida, Malaysia, showed this to be the case, except for the iridescent colour in fruits of the Elaeocarpus angustifolius Blume (Elaeocarpaceae). There I show that the colour is caused by a remarkable structure in the epidermis, and provide evidence for its selective advantage.
-
Ultrastructural Basis and Developmental Control of Blue Iridescence in Selaginella Leaves
by Charles Hebant and David W. Lee
in American Journal of Botany, 71.2, 1984
ABSTRACT
The iridescent blue color of several Selaginella species is caused by a physical effect, thin-film interference. Predictions for a model film have been confirmed by electron microscopy of S. willdenowii and S. uncinata. For the latter species iridescence contributes to leaf absorption at wavelengths above 450 nm and develops in environments enriched with far-red (730 nm) light. This evidence supports the involvement of phytochrome in the developmental control of iridescence.
-
Physical basis and ecological significance of iridescence in blue plants
by D. W. Lee and J. B. Lowry
in Nature 254, 1975
Many terrestrial plants of lowland tropical rainforests exhibit a conspicuous blue-green iridescence on their leaves—Richards has observed these plants in Africa, South America, and South-east Asia. We have seen many species of blue plants on the rainforest floor in Malaya, from diverse groups including the ferns, Selaginella, and flowering plants. In Malaya the most spectacular and most common iridescent blue plant in Selaginella willdenovii (Desv.) Spring, also frequently cultivated in greenhouses. All comments on the iridescent colour refer back to the observation of Stahl who reported the presence of reflection granules in the epidermal cells of S. wildenovii.